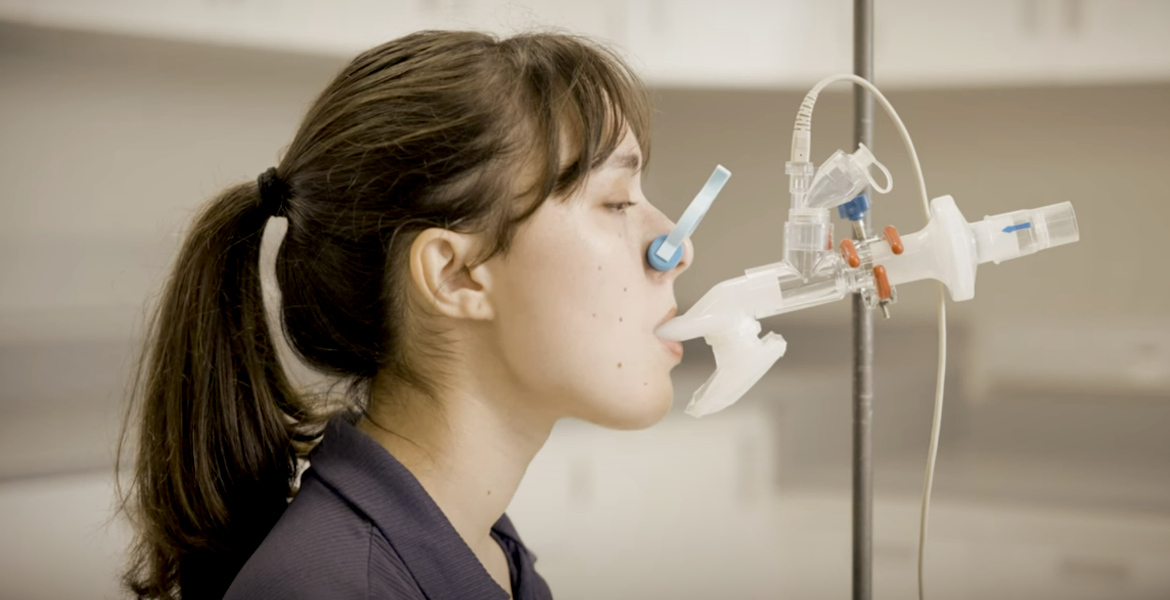
The COVID vaccine AeroVax is not to be injected – but inhaled according to a method that will be tested in Canada with government funding. This is despite previous vaccines being linked to millions of reported side effects and vaccine injuries, including heart muscle inflammation, blood clots, and sudden deaths.
At McMaster University in Ontario, Canada, researchers have launched a phase 2 study of a new COVID vaccine that is not administered by injection but is instead inhaled.
The vaccine, named ChAd-triCoV/Mac, will now be tested on 350 participants from across the country, with $8 million in government funding, and researchers say they hope the new vaccine will provide stronger protection against infection than previous injection-based vaccines.
– While the current, needle-based COVID-19 vaccines have prevented a tremendous amount of death and hospitalization, they haven’t really changed a lot of people’s experience with getting recurrent infections, claims Fiona Smaill, professor in the Department of Pathology and Molecular Medicine and one of the study's leaders.
– We’re looking to change that by providing robust protection directly at the site of infection, she declares.
Targets three virus proteins
The new vaccine differs from previous COVID vaccines in several ways. In addition to being administered as an aerosol inhalation, it targets three different proteins in the structure of the SARS-CoV-2 virus. According to the researchers, this should improve protection against any future variants of COVID.
When a vaccine is inhaled, the body's immune system reacts differently than when it is injected, which McMasters claims is more effective in preventing the infection itself.
Results from preclinical studies, together with unpublished data from the phase 1 study, suggest that the inhaled vaccine elicits a stronger immune response than traditional injections because it targets the airways where the virus first enters the body.
For those already vaccinated – but not recently ill
AeroVax is intended for people who have already received at least three doses of an mRNA vaccine. To participate in the study, participants must not have had COVID-19 or been vaccinated in the three months prior to registration. Participants must be between 18 and 65 years of age, free of lung disease, and able to attend all on-site testing sessions.
The study includes 350 participants from across Canada. Two-thirds will receive the vaccine and one-third a placebo. None of the participants know which group they belong to, which the researchers say is crucial for an objective evaluation.
– Randomization allows for objective comparison between those who received the vaccine and those who didn’t, which can tell us a lot about the level of protection the vaccine could provide and its side effects, Smaill continues.
Canadian Government Begins Testing Inhaled Covid mRNA 'AeroVax'
"The Canadian government has begun ramping up testing for a chilling new Covid mRNA “AeroVax” that seeks to overcome “vaccine hesitancy” by using aerosols to “vaccinate” the general public.
Unlike traditional… pic.twitter.com/o7j6e8U8Fp
— Robin Monotti (@robinmonotti) May 2, 2025
"Ensures the safety of participants"
The study is led by researchers at McMaster's Michael G. DeGroote Institute for Infectious Disease Research, and all development – from laboratory design to testing – is taking place in Canada.
– Every medicine or vaccine that we use and trust today has at one point gone through similar clinical trials processes, says Matthew Miller, director of the institute and a member of the research team.
– This is a highly regulated process with extensive oversight that ensures the safety of participants and will generate critical data to inform the next steps in development, he adds.
If the results are promising, the researchers plan to move on to a Phase 3 study with a larger group of participants – a step toward approval and market introduction.

Millions of side effects
In this context, it is worth mentioning that since the rollout of COVID-19 vaccines began, millions of side effects and vaccine injuries, including deaths, have been reported globally – ranging from fever and nerve damage to blood clots, heart muscle inflammation, and sudden death.
Young men have shown an increased risk of heart inflammation, which has led to certain vaccines being withdrawn or restricted in several countries, and many have come forward to testify about long-term vaccine injuries that affect their ability to work and their quality of life – but their stories have been silenced or dismissed by the healthcare system.
Critics argue that authorities and vaccine manufacturers prioritized rapid distribution over transparency and safety, and that the long-term effects are still very poorly understood.
The vaccine is also believed to have had virtually no effect on the spread of infection, and US health authorities were eventually forced to admit that vaccinated people could carry as much of the virus as unvaccinated people. In Sweden, too, healthcare providers concluded that the vaccine did not stop the spread of COVID-19.